Have you ever wondered whether nitrogen is heavier than oxygen? These two elements make up the majority of Earth's atmosphere, and understanding their properties is not only fascinating but also crucial for fields like chemistry, physics, and environmental science. Nitrogen and oxygen are essential for life as we know it, yet their differences in weight and behavior often spark curiosity. In this article, we’ll dive deep into the science behind these two elements, exploring their atomic structures, molecular weights, and real-world implications.
Nitrogen and oxygen are the most abundant gases in the Earth’s atmosphere, accounting for approximately 78% and 21%, respectively. Despite their prevalence, many people are unaware of the fundamental differences between these two elements, including their molecular weights. This article will address the question, "Is nitrogen heavier than oxygen?" in detail, providing a comprehensive understanding of their properties and significance. By the end of this piece, you’ll have a clear answer and a deeper appreciation for the role these gases play in our lives.
Whether you're a student, a science enthusiast, or simply curious about the world around you, this article is designed to provide valuable insights. We’ll cover everything from the basics of atomic and molecular weights to the practical applications of nitrogen and oxygen in various industries. So, let’s embark on this scientific journey and uncover the truth about these two vital elements.
Read also:Bocil Bokep
Table of Contents
- Understanding the Atomic Structure of Nitrogen and Oxygen
- What is Molecular Weight and Why Does it Matter?
- Comparing Nitrogen and Oxygen: Which is Heavier?
- Real-World Applications of Nitrogen and Oxygen
- The Environmental Impact of Nitrogen and Oxygen
- Industrial Uses of Nitrogen and Oxygen
- Health Implications of Nitrogen and Oxygen
- The Scientific Significance of Nitrogen and Oxygen
- Common Myths and Misconceptions
- Conclusion: Why Understanding Nitrogen and Oxygen Matters
Understanding the Atomic Structure of Nitrogen and Oxygen
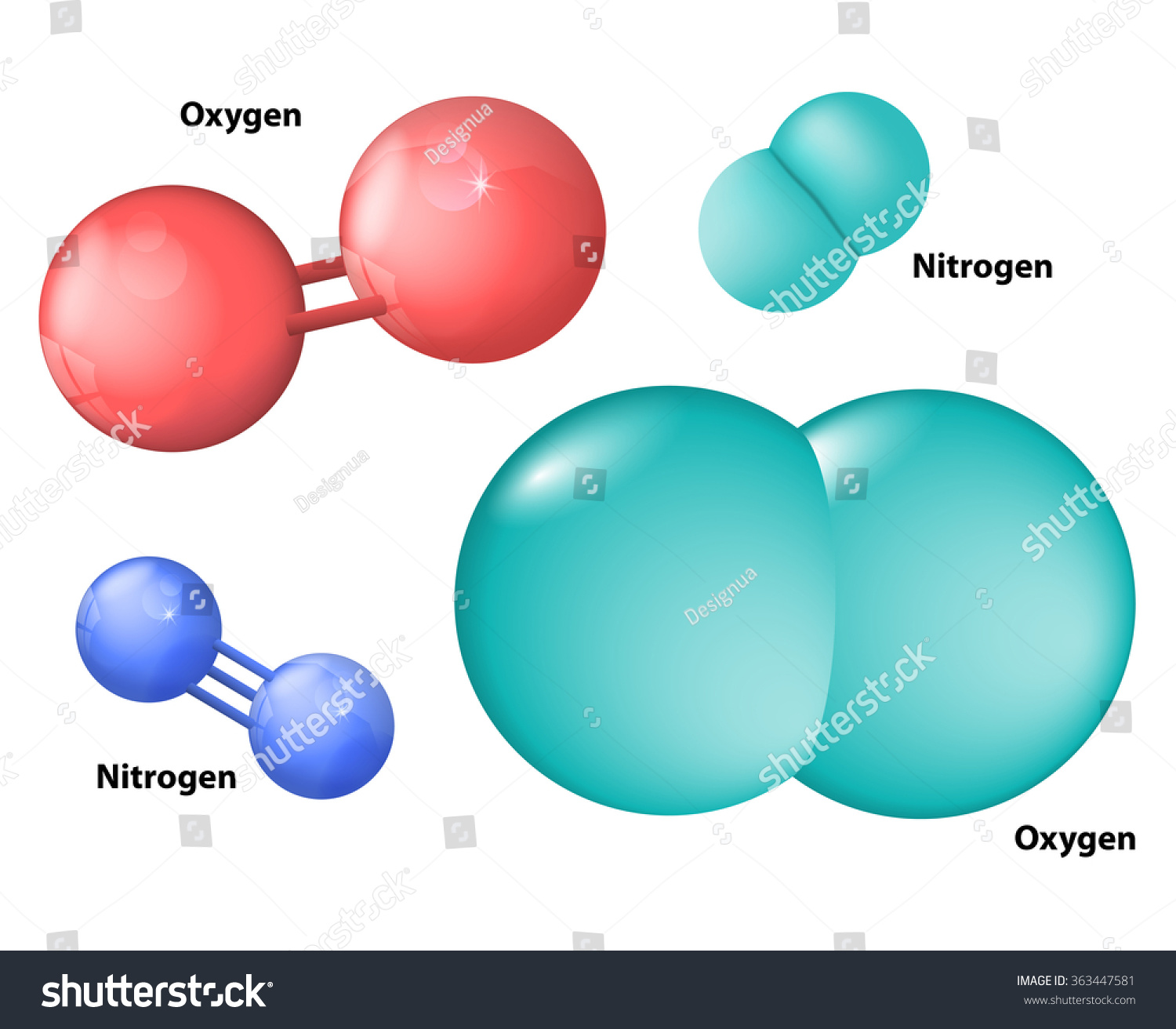
To determine whether nitrogen is heavier than oxygen, we must first understand their atomic structures. Atoms are the building blocks of all matter, and each element has a unique atomic structure defined by its number of protons, neutrons, and electrons. Nitrogen, with the atomic symbol N, has an atomic number of 7, meaning it has 7 protons in its nucleus. Oxygen, represented by the symbol O, has an atomic number of 8, indicating 8 protons in its nucleus.
The mass of an atom is primarily determined by its protons and neutrons, as electrons contribute negligibly to the overall mass. Nitrogen typically has 7 neutrons in its most common isotope, giving it an atomic mass of approximately 14 atomic mass units (amu). Oxygen, on the other hand, has 8 neutrons in its most abundant isotope, resulting in an atomic mass of about 16 amu. This difference in atomic mass is a key factor in determining whether nitrogen is heavier than oxygen.
Why Atomic Structure Matters
Understanding atomic structure is crucial because it influences how elements interact with one another. For example, nitrogen and oxygen form diatomic molecules (N₂ and O₂) in their natural states. These molecules play critical roles in processes like respiration, combustion, and atmospheric chemistry. By examining their atomic structures, we can better understand their behavior and properties.
What is Molecular Weight and Why Does it Matter?
Molecular weight refers to the total mass of a molecule, calculated by summing the atomic masses of all the atoms in the molecule. For diatomic molecules like nitrogen (N₂) and oxygen (O₂), the molecular weight is simply twice the atomic mass of the respective element. Nitrogen’s molecular weight is approximately 28 amu (14 amu × 2), while oxygen’s molecular weight is roughly 32 amu (16 amu × 2).
Why does molecular weight matter? It affects how gases behave in the atmosphere, including their density and diffusion rates. Heavier molecules tend to settle lower in the atmosphere, while lighter molecules rise. This principle is essential for understanding atmospheric composition and dynamics.
Factors Influencing Molecular Weight
Several factors can influence molecular weight, including isotopic variations and molecular bonding. For instance, isotopes of nitrogen and oxygen exist with different numbers of neutrons, leading to slight variations in molecular weight. These variations, though small, can have significant implications in scientific research and industrial applications.
Read also:Remoteiot Vpc Ssh Raspberry Pi Aws A Comprehensive Guide
Comparing Nitrogen and Oxygen: Which is Heavier?
Now that we understand the concept of molecular weight, let’s directly compare nitrogen and oxygen. As previously mentioned, nitrogen has a molecular weight of approximately 28 amu, while oxygen weighs in at about 32 amu. This means that oxygen is indeed heavier than nitrogen on a molecular level.
However, it’s important to note that the difference in weight doesn’t significantly affect their distribution in the atmosphere. Both gases are well-mixed due to constant atmospheric motion, such as wind and turbulence. Nevertheless, the molecular weight difference becomes relevant in specific scenarios, such as gas separation processes or high-altitude atmospheric studies.
Why Oxygen is Heavier
The reason oxygen is heavier lies in its atomic structure. With more protons and neutrons in its nucleus, oxygen atoms are inherently denser than nitrogen atoms. When these atoms form diatomic molecules, the difference in molecular weight becomes even more pronounced.
Real-World Applications of Nitrogen and Oxygen
Nitrogen and oxygen have numerous real-world applications that leverage their unique properties. For example, nitrogen is widely used in the food industry as a preservative and in the production of fertilizers. Its inert nature makes it ideal for creating controlled environments, such as in the packaging of perishable goods.
Oxygen, on the other hand, is essential for medical applications, including oxygen therapy and life support systems. It’s also used in industrial processes like steel production and wastewater treatment. The difference in molecular weight between nitrogen and oxygen plays a role in how these gases are stored, transported, and utilized.
Applications in Everyday Life
- Nitrogen is used in tire inflation to reduce oxidation and maintain pressure stability.
- Oxygen is critical for combustion processes, including those in internal combustion engines.
- Both gases are used in welding and metalworking, with oxygen acting as an oxidizer and nitrogen as a shielding gas.
The Environmental Impact of Nitrogen and Oxygen
Nitrogen and oxygen play vital roles in maintaining the balance of Earth’s ecosystems. Nitrogen, for instance, is a key component of amino acids and nucleic acids, which are essential for life. However, excessive nitrogen in the form of nitrates can lead to environmental issues like water pollution and eutrophication.
Oxygen is crucial for aerobic organisms, including humans, as it supports respiration. However, oxygen depletion in water bodies, known as hypoxia, can have devastating effects on aquatic life. Understanding the molecular weight and behavior of these gases helps scientists address environmental challenges and develop sustainable solutions.
Addressing Environmental Challenges
Efforts to mitigate nitrogen pollution include improving agricultural practices and developing technologies for nitrogen removal in wastewater treatment. Similarly, oxygen enrichment strategies are employed to combat hypoxia in aquatic ecosystems. These initiatives highlight the importance of understanding the properties of nitrogen and oxygen.
Industrial Uses of Nitrogen and Oxygen
In the industrial sector, nitrogen and oxygen are indispensable. Nitrogen is used in the production of ammonia, a key ingredient in fertilizers, and as a refrigerant in cryogenic applications. Its low reactivity makes it ideal for preventing oxidation and contamination in various processes.
Oxygen is widely used in steelmaking, where it enhances the efficiency of the combustion process. It’s also employed in the chemical industry for the production of acids, plastics, and other materials. The difference in molecular weight between nitrogen and oxygen influences their roles in these applications, as heavier gases often require specialized handling and storage.
Key Industrial Applications
- Nitrogen is used in the electronics industry for purging and inerting processes.
- Oxygen is critical for oxy-fuel cutting and welding in metal fabrication.
- Both gases are used in the aerospace industry for propulsion and life support systems.
Health Implications of Nitrogen and Oxygen
While nitrogen and oxygen are essential for life, they can also pose health risks under certain conditions. For example, exposure to high concentrations of nitrogen can lead to asphyxiation by displacing oxygen in the air. This is particularly dangerous in confined spaces where nitrogen is used for inerting.
Oxygen, while vital for respiration, can be harmful in excessive amounts. Prolonged exposure to high oxygen concentrations can cause oxidative stress and damage to tissues. Understanding the properties of these gases, including their molecular weights, is crucial for ensuring safe handling and use.
Safety Measures
To mitigate health risks, industries implement strict safety protocols when working with nitrogen and oxygen. These include proper ventilation, monitoring oxygen levels, and using personal protective equipment. Public awareness campaigns also emphasize the importance of handling these gases responsibly.
The Scientific Significance of Nitrogen and Oxygen
Nitrogen and oxygen are subjects of extensive scientific research due to their fundamental roles in chemistry, biology, and environmental science. Studies on their molecular weights and interactions have led to breakthroughs in fields like atmospheric science and materials engineering.
For instance, understanding the behavior of nitrogen and oxygen in the atmosphere has improved climate models and predictions. Research on their properties has also advanced technologies for gas separation, storage, and utilization. These scientific endeavors underscore the importance of studying these elements in detail.
Recent Discoveries
Recent studies have explored the use of nitrogen and oxygen in novel applications, such as the development of nitrogen-based fuels and oxygen-enriched materials. These innovations highlight the ongoing relevance of these gases in scientific and industrial contexts.
Common Myths and Misconceptions
Despite their prevalence, nitrogen and oxygen are often misunderstood. One common myth is that nitrogen is heavier than oxygen, which, as we’ve established, is incorrect. Another misconception is that nitrogen is inert and therefore harmless, overlooking its potential to cause asphyxiation in high concentrations.
Addressing these myths is essential for promoting accurate knowledge and safe practices. By dispelling misconceptions, we can foster a better understanding of these critical elements and their roles in our lives.
Debunking Myths
- Nitrogen is lighter than oxygen, not heavier.
- Nitrogen can pose health risks in confined spaces despite its inert nature.
- Oxygen is not always beneficial in high concentrations; it can cause oxidative stress.
Conclusion: Why Understanding Nitrogen and Oxygen Matters
In conclusion, oxygen is indeed heavier than nitrogen, with a molecular weight of approximately 32 amu compared to nitrogen’s 28 amu. This difference, though seemingly small, has significant implications in various fields, from atmospheric science to industrial applications. Understanding the properties of these gases is not only fascinating but also essential for addressing environmental, health, and technological challenges.
We encourage you to share your thoughts on this topic in the comments below. Did you find this article informative? If so, please share it with others who might benefit from this knowledge. For more in-depth articles on science and technology, explore our website and stay tuned for future updates. Together, let’s continue to uncover the mysteries of the world around us!