Understanding how to get atomic mass is essential for anyone studying chemistry or physics. Atomic mass plays a crucial role in determining the properties of elements and compounds, making it a foundational concept in science. Whether you are a student, researcher, or simply curious about the natural world, mastering this topic will deepen your knowledge of the building blocks of matter. In this article, we will explore the concept of atomic mass, how it is calculated, and why it matters in various scientific disciplines.
Atomic mass is a weighted average of the masses of all isotopes of an element, taking into account their natural abundance. This value is expressed in atomic mass units (amu) and is a key component of the periodic table. Understanding how to calculate and interpret atomic mass is vital for anyone working in fields such as chemistry, biology, and engineering. By the end of this article, you will have a clear understanding of how to determine atomic mass and its significance in scientific research.
This guide will take you through the step-by-step process of calculating atomic mass, provide real-world examples, and discuss its applications. We will also address common misconceptions and answer frequently asked questions to ensure you have a comprehensive understanding of the topic. Whether you are preparing for an exam or conducting research, this article will equip you with the knowledge you need to confidently work with atomic mass.
Read also:Is Ciara Pregnant In 2025 Everything You Need To Know
Table of Contents
What is Atomic Mass?
Atomic mass refers to the mass of an atom, typically expressed in atomic mass units (amu). It is a weighted average of the masses of all isotopes of an element, taking into account their natural abundance. This value is crucial for understanding the behavior of elements and compounds in chemical reactions.
Definition and Explanation
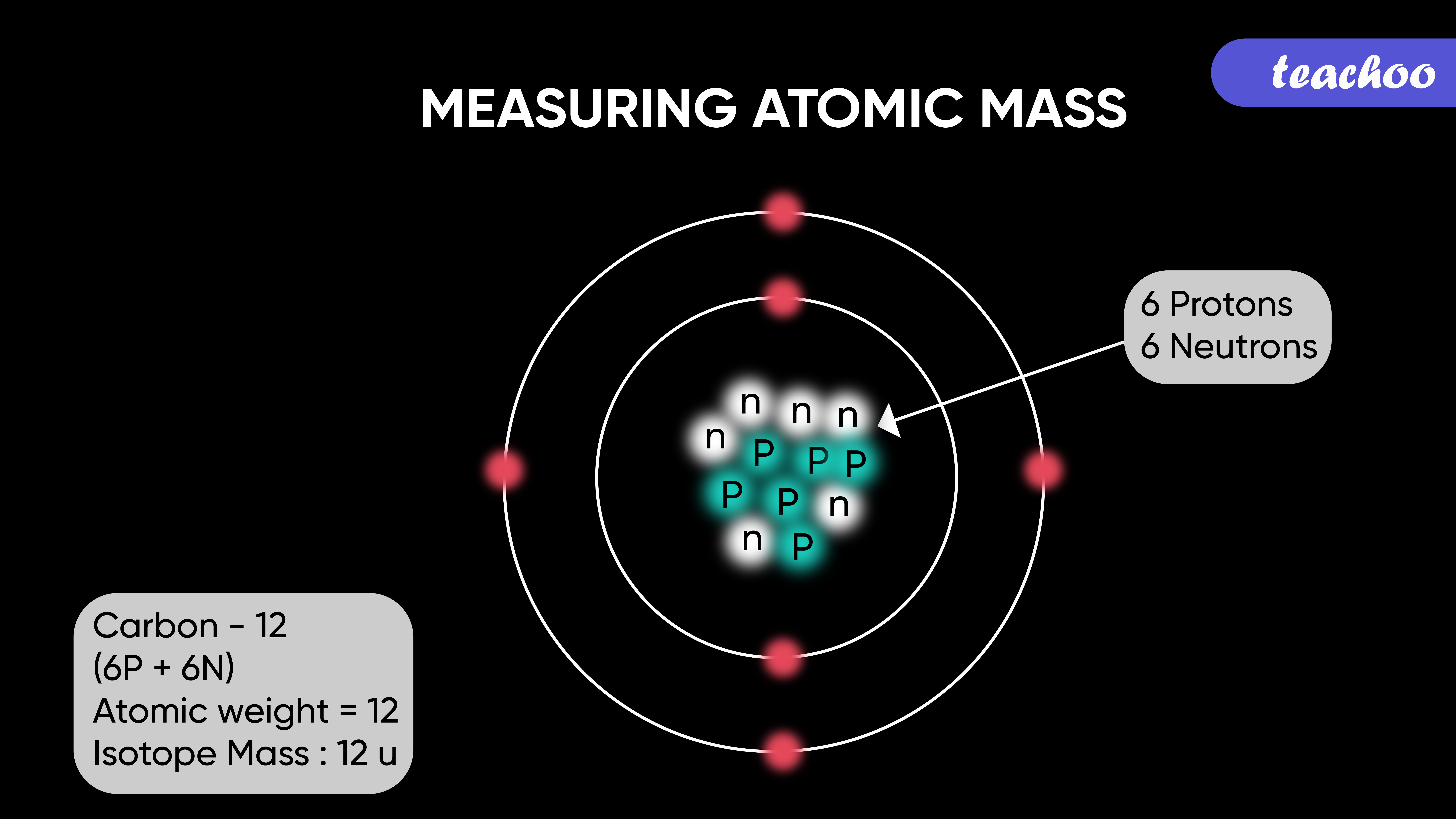
The atomic mass of an element is calculated by summing the masses of protons and neutrons in its nucleus. Electrons contribute negligibly to the overall mass, as their mass is significantly smaller than that of protons and neutrons. The standard unit for atomic mass is the atomic mass unit (amu), which is defined as one-twelfth the mass of a carbon-12 atom.
Isotopes and Their Role in Atomic Mass
Isotopes are atoms of the same element that have different numbers of neutrons. Since isotopes of an element have varying masses, the atomic mass listed on the periodic table is a weighted average of all naturally occurring isotopes. For example, chlorine has two stable isotopes: chlorine-35 and chlorine-37. The atomic mass of chlorine is calculated based on the relative abundance of these isotopes.
How to Calculate Atomic Mass
Calculating atomic mass involves determining the weighted average of the masses of all isotopes of an element. This process requires knowledge of the isotopic masses and their natural abundances. Below is a step-by-step guide to help you calculate atomic mass accurately.
Step 1: Identify the Isotopes
The first step in calculating atomic mass is to identify the isotopes of the element in question. For example, carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. Each isotope has a unique mass due to the varying number of neutrons in its nucleus.
Step 2: Determine Isotopic Masses
Next, determine the mass of each isotope. Isotopic masses are typically measured using mass spectrometry, a technique that separates isotopes based on their mass-to-charge ratio. These masses are often expressed in atomic mass units (amu).
Read also:Aishah Sofey Exploring The Life And Achievements Of A Rising Star
Step 3: Find Natural Abundance
The natural abundance of each isotope is expressed as a percentage. For example, chlorine-35 has a natural abundance of approximately 75%, while chlorine-37 accounts for the remaining 25%. These percentages are essential for calculating the weighted average.
Step 4: Apply the Formula
The formula for calculating atomic mass is as follows:
Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + ...
For example, the atomic mass of chlorine can be calculated as:
Atomic Mass = (34.96885 amu × 0.7578) + (36.96590 amu × 0.2422) = 35.453 amu
Step 5: Verify Your Calculation
After performing the calculation, verify your result by comparing it to the atomic mass listed on the periodic table. This ensures accuracy and helps identify any potential errors in your computation.
Why Atomic Mass Matters
Atomic mass is a fundamental concept in chemistry and physics, influencing a wide range of scientific fields. Understanding its importance can help you appreciate its role in both theoretical and practical applications.
Chemical Reactions and Stoichiometry
In chemical reactions, atomic mass is used to determine the molar mass of compounds, which is essential for stoichiometric calculations. By knowing the atomic mass of each element in a compound, chemists can calculate the amount of reactants and products involved in a reaction.
Periodic Table Organization
The periodic table is organized based on atomic number and atomic mass. Elements are arranged in order of increasing atomic number, and their atomic masses provide insights into their chemical properties and behavior.
Biological Applications
Atomic mass is also relevant in biology, particularly in the study of isotopes. For example, carbon-14 is used in radiocarbon dating to estimate the age of archaeological artifacts. Understanding isotopic masses helps scientists interpret data and draw accurate conclusions.
Real-World Applications of Atomic Mass
Atomic mass has numerous practical applications across various fields, from medicine to environmental science. Below are some examples of how atomic mass is used in real-world scenarios.
Pharmaceutical Development
In the pharmaceutical industry, atomic mass is used to determine the molecular weight of drugs. This information is critical for ensuring the correct dosage and understanding how drugs interact with the body.
Environmental Monitoring
Isotopic analysis, which relies on atomic mass, is used to monitor environmental changes. For example, scientists study the isotopic composition of water to track pollution sources and assess the health of ecosystems.
Nuclear Energy
Atomic mass plays a key role in nuclear energy production. The mass difference between reactants and products in nuclear reactions determines the amount of energy released, as described by Einstein's equation, E=mc².
Common Misconceptions About Atomic Mass
Despite its importance, atomic mass is often misunderstood. Below are some common misconceptions and clarifications to help you avoid confusion.
Misconception 1: Atomic Mass Equals Atomic Weight
While atomic mass and atomic weight are related, they are not the same. Atomic mass refers to the mass of a single atom, while atomic weight is the weighted average of all isotopes of an element.
Misconception 2: Electrons Contribute Significantly to Atomic Mass
Electrons have a negligible impact on atomic mass due to their extremely small size compared to protons and neutrons. The mass of an atom is primarily determined by its nucleus.
Misconception 3: Atomic Mass is Always a Whole Number
Atomic mass is rarely a whole number because it is a weighted average of isotopic masses. For example, the atomic mass of chlorine is approximately 35.45 amu, reflecting the contributions of its isotopes.
Frequently Asked Questions
Here are some common questions about atomic mass and their answers:
What is the Difference Between Atomic Mass and Molar Mass?
Atomic mass refers to the mass of a single atom, while molar mass is the mass of one mole of a substance. Molar mass is expressed in grams per mole (g/mol) and is numerically equal to the atomic mass in amu.
Why is Atomic Mass Important in Chemistry?
Atomic mass is essential for understanding chemical reactions, determining molecular weights, and organizing the periodic table. It provides a foundation for many calculations in chemistry.
How Do Isotopes Affect Atomic Mass?
Isotopes affect atomic mass by contributing varying masses and natural abundances. The weighted average of these isotopes determines the atomic mass listed on the periodic table.
Conclusion
In this article, we have explored the concept of atomic mass, how to calculate it, and its significance in various scientific fields. Understanding atomic mass is crucial for anyone studying chemistry, physics, or biology, as it provides insights into the behavior of elements and compounds. By mastering the steps to calculate atomic mass and recognizing its real-world applications, you can enhance your scientific knowledge and problem-solving skills.
We encourage you to apply this knowledge in your studies or research and share your thoughts in the comments below. If you found this article helpful, please share it with others who may benefit from it. For more in-depth guides on scientific topics, explore our website and continue your learning journey today.