Hypochlorous acid is a fascinating chemical compound with a wide range of applications in various industries. This weak acid, often recognized for its role in disinfection and sanitation, has a simple yet significant chemical formula that forms the basis of its properties. Understanding its composition and functionality is essential for anyone involved in chemistry, healthcare, or even household cleaning. In this article, we will explore the chemical formula for hypochlorous acid, its properties, uses, and much more.
Before diving into the details, it’s important to note that hypochlorous acid is not just another chemical compound. It is a key player in maintaining hygiene and safety in both industrial and domestic settings. Its ability to act as a powerful oxidizing agent makes it indispensable in water treatment, medical sterilization, and even skincare products. This article will provide a thorough analysis of its chemical structure, properties, and applications, ensuring you have all the information you need.
Whether you're a student, a professional, or simply curious about this compound, this guide will equip you with the knowledge to understand hypochlorous acid better. We will also explore its safety considerations, environmental impact, and future potential in various fields. Let’s begin by breaking down the chemical formula of hypochlorous acid and understanding its significance.
Read also:Natalie Winters A Comprehensive Guide To Her Life Career And Achievements
Table of Contents
- The Chemical Formula of Hypochlorous Acid
- Key Properties of Hypochlorous Acid
- Applications and Uses of Hypochlorous Acid
- How Hypochlorous Acid is Produced
- Safety Considerations When Handling Hypochlorous Acid
- Environmental Impact of Hypochlorous Acid
- The Future of Hypochlorous Acid in Various Industries
- Hypochlorous Acid vs. Other Disinfectants
- Frequently Asked Questions About Hypochlorous Acid
- Conclusion
The Chemical Formula of Hypochlorous Acid
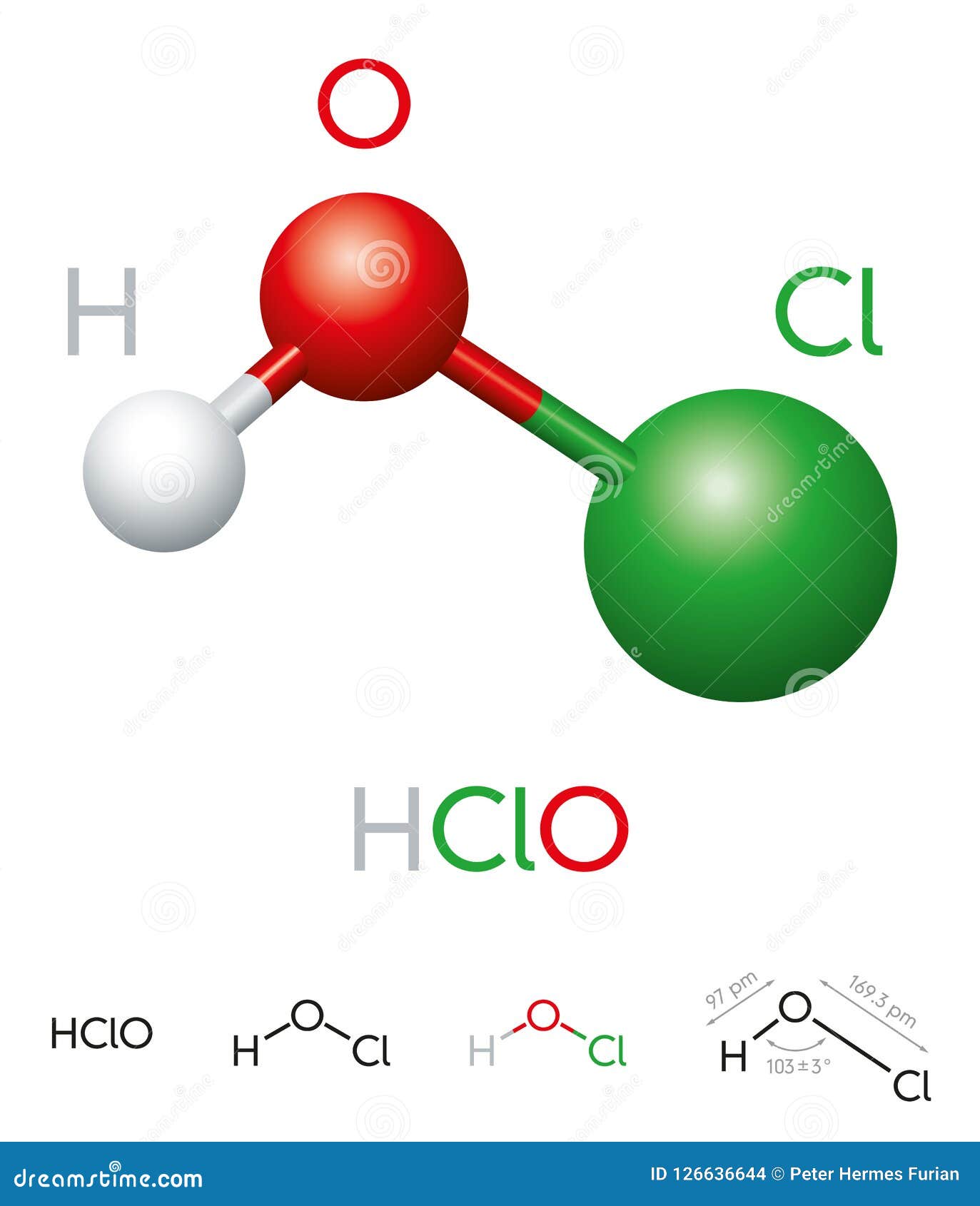
The chemical formula for hypochlorous acid is HOCl. This formula represents a molecule composed of one hydrogen (H) atom, one oxygen (O) atom, and one chlorine (Cl) atom. The arrangement of these atoms gives hypochlorous acid its unique properties, making it a weak acid with strong oxidizing capabilities.
Understanding the Components of HOCl
- Hydrogen (H): The hydrogen atom contributes to the acidic nature of the compound.
- Oxygen (O): The oxygen atom forms a bond with both hydrogen and chlorine, stabilizing the molecule.
- Chlorine (Cl): The chlorine atom is responsible for the compound's disinfectant properties.
The HOCl molecule exists in equilibrium with its dissociated ions in water, primarily hypochlorite (OCl⁻). This equilibrium is crucial for its effectiveness as a disinfectant and sanitizer.
Key Properties of Hypochlorous Acid
Hypochlorous acid exhibits several unique properties that make it highly valuable in various applications. Below, we’ll explore its physical, chemical, and biological characteristics.
Physical Properties
- Appearance: Hypochlorous acid is typically found in aqueous solutions, appearing as a colorless liquid.
- Odor: It has a slightly chlorine-like smell, which is noticeable in higher concentrations.
- Solubility: HOCl is highly soluble in water, which enhances its usability in liquid-based applications.
Chemical Properties
- Oxidizing Agent: Hypochlorous acid is a powerful oxidizer, capable of breaking down organic and inorganic contaminants.
- Weak Acid: Despite its oxidizing strength, HOCl is classified as a weak acid due to its limited dissociation in water.
- Stability: The compound is relatively unstable, especially in the presence of light and heat, which can cause it to decompose into oxygen and hydrochloric acid.
Applications and Uses of Hypochlorous Acid
Hypochlorous acid is widely used across multiple industries due to its versatility and effectiveness. Below are some of the most common applications.
Water Treatment
One of the primary uses of hypochlorous acid is in water treatment. It is employed to disinfect drinking water, swimming pools, and wastewater. Its ability to kill bacteria, viruses, and other pathogens makes it an essential component in ensuring water safety.
Healthcare and Medical Applications
In healthcare, hypochlorous acid is used for sterilizing medical equipment, wound care, and surface disinfection. Its non-toxic nature and effectiveness against a broad spectrum of microorganisms make it a preferred choice in medical settings.
Read also:Buscar Kid And Mom A Comprehensive Guide To Family Travel And Adventure
Household Cleaning
Hypochlorous acid is also found in household cleaning products, such as disinfectant sprays and sanitizers. It is particularly valued for its ability to clean surfaces without leaving harmful residues.
How Hypochlorous Acid is Produced
Hypochlorous acid is typically produced through the electrolysis of a saline solution. This process involves passing an electric current through a solution of salt (sodium chloride) and water, which generates hypochlorous acid and sodium hydroxide as byproducts.
Steps in the Production Process
- Electrolysis: The saline solution is subjected to an electric current, causing the formation of HOCl.
- Separation: The resulting solution is then separated to isolate the hypochlorous acid.
- Stabilization: To enhance its shelf life, stabilizing agents may be added to the solution.
Safety Considerations When Handling Hypochlorous Acid
While hypochlorous acid is generally considered safe, certain precautions must be taken when handling it. Below are some important safety tips.
Storage Guidelines
- Temperature Control: Store hypochlorous acid in a cool, dark place to prevent decomposition.
- Container Material: Use containers made of materials resistant to corrosion, such as glass or high-density polyethylene.
Handling Precautions
- Ventilation: Ensure proper ventilation when using hypochlorous acid to avoid inhalation of fumes.
- Protective Gear: Wear gloves and safety goggles to protect your skin and eyes from accidental exposure.
Environmental Impact of Hypochlorous Acid
Hypochlorous acid has a relatively low environmental impact compared to other disinfectants. However, its decomposition products, such as chlorine gas, can pose risks if not managed properly.
Positive Environmental Contributions
- Biodegradability: Hypochlorous acid breaks down into harmless substances like water and oxygen.
- Reduced Chemical Residues: Its use minimizes the accumulation of harmful residues in ecosystems.
Potential Risks
- Chlorine Byproducts: Improper handling can lead to the release of chlorine gas, which is harmful to both humans and the environment.
- Water Contamination: Overuse in water treatment can lead to the formation of chlorinated organic compounds.
The Future of Hypochlorous Acid in Various Industries
As research continues, the potential applications of hypochlorous acid are expanding. Innovations in its production and stabilization are making it more accessible and effective for a broader range of uses.
Emerging Trends
- Green Chemistry: Hypochlorous acid is gaining traction as a sustainable alternative to traditional disinfectants.
- Skincare Products: Its antimicrobial properties are being explored for use in acne treatments and wound healing.
Hypochlorous Acid vs. Other Disinfectants
When compared to other disinfectants like bleach (sodium hypochlorite) and hydrogen peroxide, hypochlorous acid stands out for its safety and effectiveness.
Advantages of Hypochlorous Acid
- Non-Toxic: Unlike bleach, hypochlorous acid is safe for use on skin and surfaces.
- Fast-Acting: It provides rapid disinfection without the need for prolonged contact times.
Limitations
- Stability: Its instability requires careful storage and handling.
- Cost: Production costs can be higher compared to other disinfectants.
Frequently Asked Questions About Hypochlorous Acid
Here are some common questions people have about hypochlorous acid:
- What is the chemical formula for hypochlorous acid? The chemical formula is HOCl.
- Is hypochlorous acid safe for skin? Yes, it is generally safe and used in skincare products.
- How is hypochlorous acid used in water treatment? It is added to water to kill bacteria and viruses.
Conclusion
In conclusion, hypochlorous acid, with the chemical formula HOCl, is a remarkable compound with diverse applications. Its properties as a weak acid and powerful oxidizing agent make it indispensable in industries ranging from healthcare to water treatment. By understanding its chemical structure, properties, and uses, we can better appreciate its role in maintaining hygiene and safety.
We encourage you to share this article with others who might find it informative. If you have any questions or insights about hypochlorous acid, feel free to leave a comment below. For more articles on chemistry and related topics, explore our website today!