Hypochlorous acid is a fascinating compound that plays a crucial role in various fields, from healthcare to water treatment. Its chemical formula, HOCl, might seem simple, but its applications and properties are anything but. In this article, we will explore the formula of hypochlorous acid in detail, shedding light on its structure, uses, and significance. Whether you're a student, researcher, or simply curious about chemistry, this guide will provide you with a comprehensive understanding of hypochlorous acid and its importance in everyday life.
Hypochlorous acid is widely recognized for its antimicrobial properties and is commonly used as a disinfectant. Its ability to kill bacteria, viruses, and fungi has made it an essential component in cleaning products and medical applications. Understanding the formula of hypochlorous acid is key to appreciating its role in maintaining hygiene and public health.
In this article, we will break down the chemical structure of hypochlorous acid, explore its various applications, and discuss its environmental impact. By the end of this guide, you will have a thorough understanding of this compound and its relevance in modern science and industry.
Read also:Discovering Abby Berner The Rising Star Taking The Internet By Storm
Table of Contents
- Chemical Structure of Hypochlorous Acid
- Properties of Hypochlorous Acid
- Applications of Hypochlorous Acid
- How Hypochlorous Acid is Produced
- Environmental Impact of Hypochlorous Acid
- Safety and Handling of Hypochlorous Acid
- Health Benefits of Hypochlorous Acid
- Industrial Uses of Hypochlorous Acid
- Ongoing Research on Hypochlorous Acid
- Conclusion and Call to Action
Chemical Structure of Hypochlorous Acid
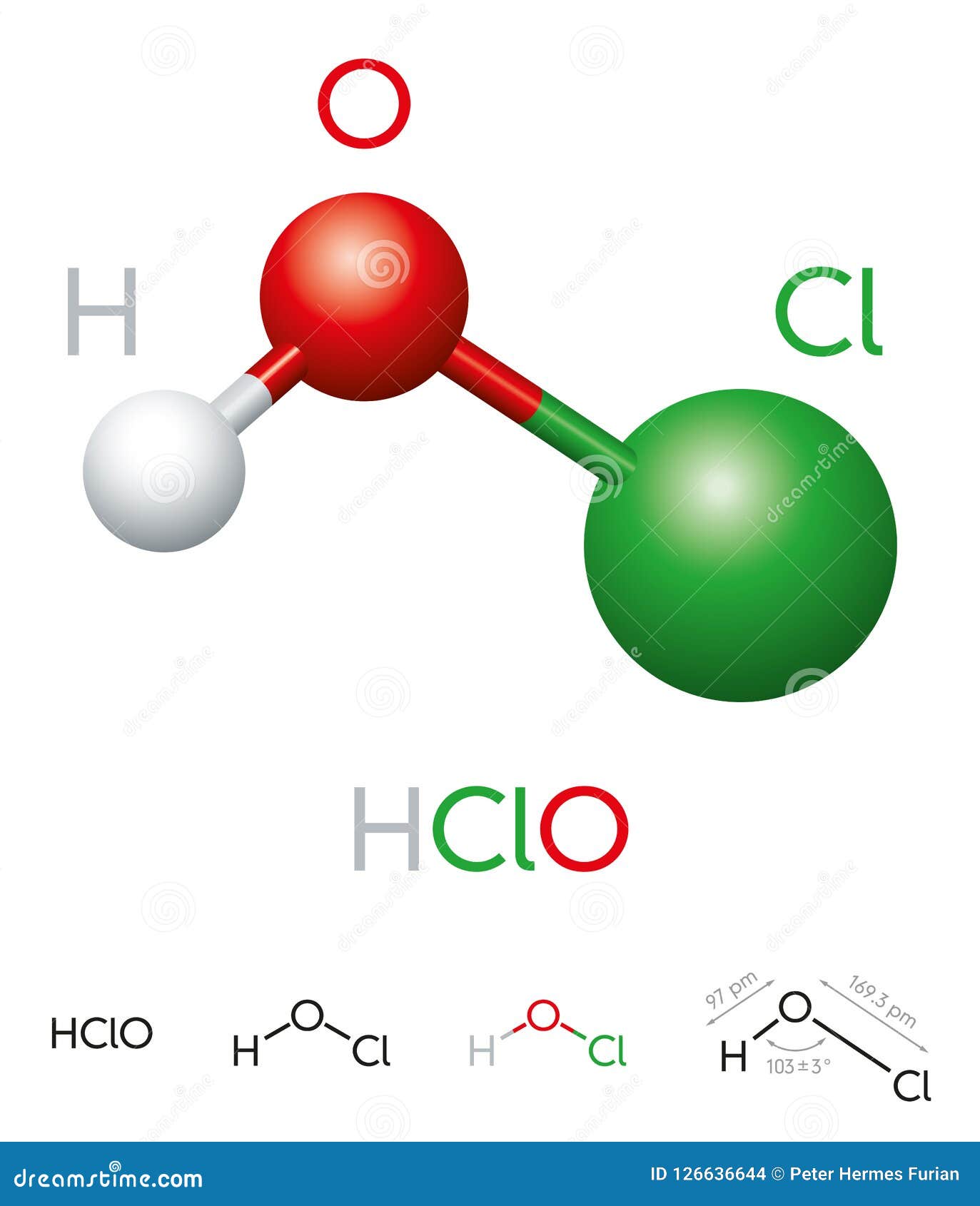
Hypochlorous acid, with the chemical formula HOCl, is a weak acid that consists of one hydrogen atom, one oxygen atom, and one chlorine atom. The molecular structure of HOCl is relatively simple, but its behavior in various chemical reactions is complex. In its pure form, hypochlorous acid is a colorless liquid that is highly unstable, which is why it is often found in solution or generated on-site.
How Hypochlorous Acid is Formed
Hypochlorous acid is typically formed when chlorine gas (Cl₂) dissolves in water. This reaction is represented by the following equation:
Cl₂ + H₂O ⇌ HOCl + HCl
In this reaction, chlorine reacts with water to produce hypochlorous acid and hydrochloric acid. The equilibrium of this reaction is influenced by factors such as pH and temperature, which we will discuss in later sections.
Properties of Hypochlorous Acid
Hypochlorous acid exhibits several unique properties that make it valuable in various applications. Below are some of its key characteristics:
- Antimicrobial Properties: Hypochlorous acid is a powerful disinfectant that can kill a wide range of microorganisms, including bacteria, viruses, and fungi.
- Weak Acidic Nature: Despite being a weak acid, it is highly effective in neutralizing harmful pathogens.
- Unstable in Pure Form: Hypochlorous acid is unstable and decomposes quickly when exposed to light or heat.
Physical Properties
Hypochlorous acid has a molecular weight of approximately 52.46 g/mol. It is highly soluble in water and exists predominantly in its dissociated form, hypochlorite ion (OCl⁻), in solutions with higher pH levels. This dissociation is crucial to its effectiveness as a disinfectant.
Read also:Chip Gaines Heart Attack Latest Updates News
Applications of Hypochlorous Acid
Hypochlorous acid is widely used in various industries due to its antimicrobial properties. Below are some of its most common applications:
- Water Treatment: It is used to disinfect drinking water and swimming pools.
- Healthcare: Hypochlorous acid is utilized in wound care and as a surface disinfectant in hospitals.
- Food Industry: It is applied to sanitize food processing equipment and surfaces.
Emerging Uses in Agriculture
Recent advancements have seen hypochlorous acid being used in agriculture to disinfect crops and prevent the spread of plant diseases. Its non-toxic nature makes it a safer alternative to traditional chemical pesticides.
How Hypochlorous Acid is Produced
The production of hypochlorous acid typically involves the electrolysis of a saline solution. This process generates hypochlorous acid on-site, ensuring its freshness and effectiveness. The electrolysis method is widely regarded as the most efficient way to produce hypochlorous acid for commercial and industrial use.
Electrolysis Process
In the electrolysis process, a saline solution (sodium chloride in water) is passed through an electrolytic cell, where it undergoes a chemical reaction to produce hypochlorous acid. This method is environmentally friendly and cost-effective, making it a preferred choice for many industries.
Environmental Impact of Hypochlorous Acid
Hypochlorous acid is considered an environmentally friendly disinfectant due to its biodegradability. Unlike many chemical disinfectants, it breaks down into harmless byproducts, such as water and salt, after use. This makes it a sustainable choice for applications where environmental impact is a concern.
Comparison with Chlorine Gas
Unlike chlorine gas, which can be hazardous to handle and store, hypochlorous acid is much safer and easier to manage. Its minimal environmental footprint has led to its increased adoption in industries seeking greener alternatives.
Safety and Handling of Hypochlorous Acid
While hypochlorous acid is generally safe to use, it is important to follow proper safety protocols to avoid potential hazards. Below are some guidelines for safe handling:
- Protective Gear: Wear gloves and goggles when handling concentrated solutions.
- Ventilation: Ensure proper ventilation in areas where hypochlorous acid is used.
- Storage: Store solutions in dark, cool places to prevent decomposition.
First Aid Measures
In case of accidental exposure, rinse the affected area with water and seek medical attention if necessary. Always refer to the product's Material Safety Data Sheet (MSDS) for detailed safety information.
Health Benefits of Hypochlorous Acid
Hypochlorous acid has gained attention in the healthcare industry for its ability to promote healing and prevent infections. It is used in wound care products to clean and disinfect wounds without causing irritation. Its gentle yet effective nature makes it suitable for use on sensitive skin.
Use in Dermatology
Dermatologists often recommend hypochlorous acid-based solutions for treating acne, eczema, and other skin conditions. Its antimicrobial properties help reduce inflammation and prevent secondary infections.
Industrial Uses of Hypochlorous Acid
Hypochlorous acid is widely used in industrial settings for its disinfectant properties. It is applied in the cleaning and sanitization of equipment, surfaces, and facilities. Its ability to kill pathogens without leaving harmful residues makes it a preferred choice in industries such as food processing, pharmaceuticals, and textiles.
Use in Textile Manufacturing
In the textile industry, hypochlorous acid is used to bleach fabrics and remove stains. Its mild nature ensures that the fabric's integrity is maintained during the bleaching process.
Ongoing Research on Hypochlorous Acid
Research on hypochlorous acid continues to uncover new applications and benefits. Scientists are exploring its potential in treating chronic wounds, developing eco-friendly cleaning products, and even combating antibiotic-resistant bacteria. These studies highlight the compound's versatility and importance in modern science.
Recent Breakthroughs
Recent studies have shown that hypochlorous acid can enhance the effectiveness of certain antibiotics, making it a valuable tool in the fight against drug-resistant infections. This discovery has opened new avenues for research and development in the healthcare sector.
Conclusion and Call to Action
In conclusion, hypochlorous acid is a remarkable compound with a wide range of applications. Its chemical formula, HOCl, represents a powerful yet gentle disinfectant that plays a vital role in maintaining hygiene and public health. From water treatment to healthcare, its uses are diverse and impactful.
We hope this article has provided you with valuable insights into the formula of hypochlorous acid and its significance. If you found this guide helpful, please consider sharing it with others who might benefit from this information. For more articles on chemistry and science, feel free to explore our website and leave your thoughts in the comments section below.