Hypochlorous acid chemical formula is a topic that has gained significant attention in recent years due to its versatile applications in various industries, including healthcare, water treatment, and sanitation. This weak acid, represented by the chemical formula HOCl, is a powerful oxidizing agent that plays a crucial role in disinfection and cleaning processes. Despite its simplicity, hypochlorous acid holds immense importance in maintaining hygiene and safety in our daily lives.
Hypochlorous acid is naturally produced by white blood cells in the human body as part of the immune response to fight infections. Its synthetic form is widely used in commercial and industrial applications, making it a compound of great interest. Understanding its chemical properties, mechanisms, and applications is essential for professionals in healthcare, chemistry, and environmental science. This article delves into the details of hypochlorous acid, exploring its chemical structure, uses, and safety considerations.
In this comprehensive guide, we will explore the science behind hypochlorous acid, its role in disinfection, and its significance in modern applications. Whether you are a student, researcher, or industry professional, this article aims to provide valuable insights into the chemical formula and properties of hypochlorous acid. By the end of this article, you will have a thorough understanding of why this compound is so widely utilized and how it can be safely and effectively applied in various fields.
Read also:Laura Loomer Unveiling The Controversial Figure Shaping Modern Discourse
- Chemical Structure of Hypochlorous Acid
- Key Properties of Hypochlorous Acid
- Mechanism of Disinfection
- Applications of Hypochlorous Acid
- How Hypochlorous Acid is Produced
- Safety Considerations and Handling
- Environmental Impact of Hypochlorous Acid
- Comparison with Other Disinfectants
- Recent Research and Developments
- Conclusion and Call to Action
Chemical Structure of Hypochlorous Acid
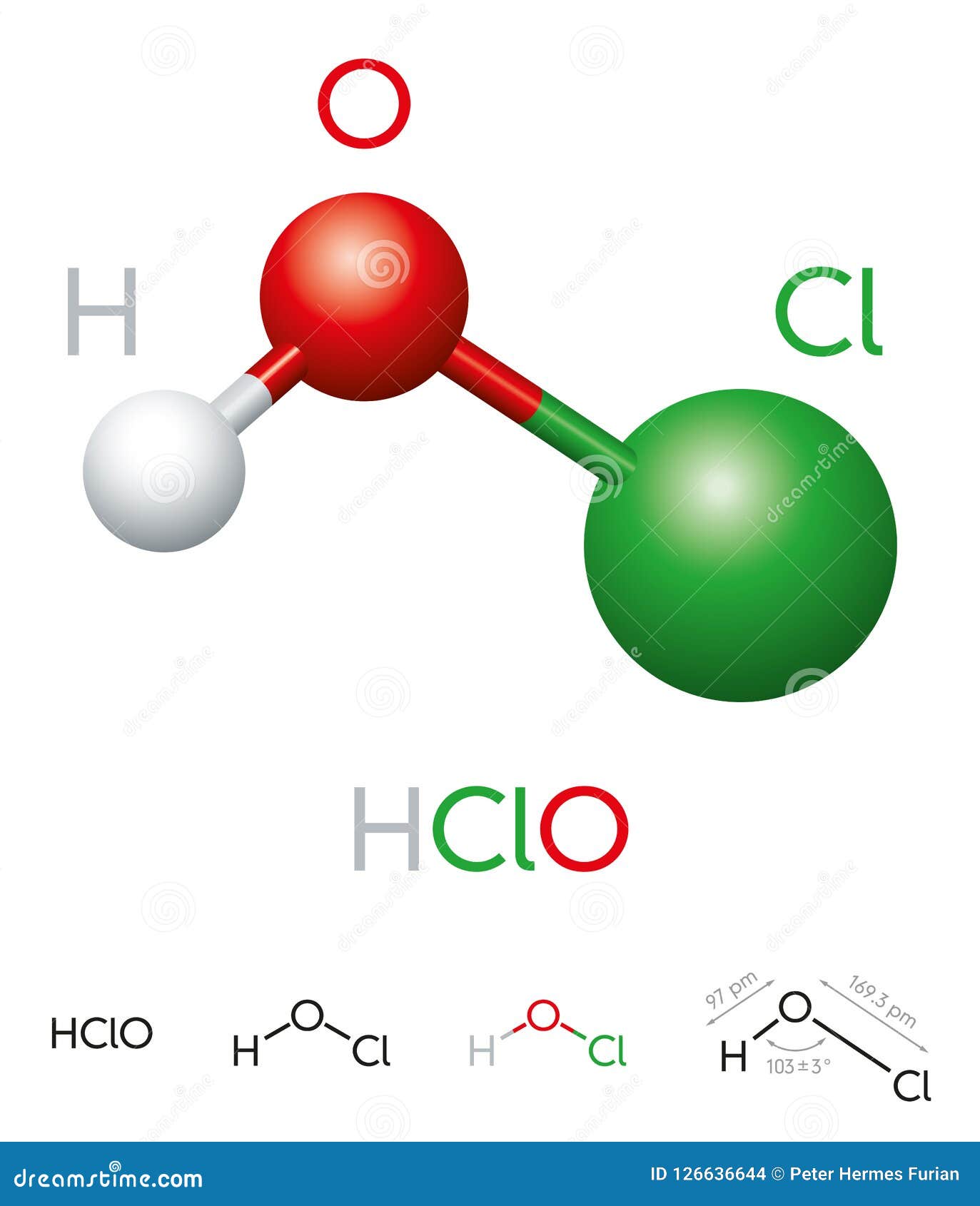
The hypochlorous acid chemical formula is HOCl, which consists of one hydrogen (H) atom, one oxygen (O) atom, and one chlorine (Cl) atom. This simple molecular structure is what gives hypochlorous acid its unique properties. The bond between oxygen and chlorine is a covalent bond, while the hydrogen atom is attached to the oxygen atom through another covalent bond. This arrangement allows hypochlorous acid to exist in equilibrium with its dissociated ions, hypochlorite (OCl⁻) and hydrogen (H⁺), in aqueous solutions.
Understanding the molecular geometry of hypochlorous acid is crucial to comprehending its behavior. The molecule has a bent shape due to the lone pair of electrons on the oxygen atom, resulting in a bond angle of approximately 103 degrees. This geometry influences its reactivity and stability, making it a potent oxidizing agent. The polarity of the molecule, caused by the electronegativity difference between oxygen and chlorine, further enhances its ability to interact with other substances.
Chemical Bonding in HOCl
The covalent bonds in hypochlorous acid are responsible for its stability and reactivity. The oxygen-chlorine bond is relatively weak compared to other covalent bonds, which explains why hypochlorous acid readily dissociates in water. This dissociation is a key factor in its effectiveness as a disinfectant, as it allows the release of reactive chlorine species that can oxidize and destroy pathogens.
Key Properties of Hypochlorous Acid
Hypochlorous acid is a weak acid with a pKa value of approximately 7.5, meaning it partially dissociates in water to form hypochlorite ions (OCl⁻) and hydrogen ions (H⁺). This property makes it highly effective in neutral and slightly acidic environments, where it exists predominantly in its molecular form (HOCl). The molecular form is more effective as a disinfectant compared to the hypochlorite ion, as it can penetrate microbial cell walls more easily.
- Weak Acid: Hypochlorous acid is classified as a weak acid, meaning it does not completely dissociate in water.
- Oxidizing Agent: It is a powerful oxidizing agent, capable of breaking down organic and inorganic contaminants.
- Stability: While effective, hypochlorous acid is relatively unstable and decomposes over time, especially when exposed to light or heat.
Reactivity and Stability
The reactivity of hypochlorous acid is influenced by factors such as pH, temperature, and the presence of other substances. For instance, at higher pH levels, hypochlorous acid dissociates more into hypochlorite ions, reducing its effectiveness as a disinfectant. Conversely, in acidic conditions, the molecular form (HOCl) predominates, enhancing its antimicrobial properties.
Mechanism of Disinfection
The hypochlorous acid chemical formula plays a pivotal role in its disinfection mechanism. When hypochlorous acid comes into contact with microorganisms, it penetrates their cell walls and disrupts vital cellular components. This process involves the oxidation of proteins, lipids, and nucleic acids, leading to the inactivation of enzymes and the eventual death of the microorganism.
Read also:Shayanna Jenkins A Comprehensive Look At Her Life Career And Influence
One of the key advantages of hypochlorous acid is its ability to act quickly and efficiently. Unlike other disinfectants, it does not leave harmful residues and is safe for use on surfaces that come into contact with food or humans. This makes it an ideal choice for applications in healthcare settings, food processing, and water treatment.
Comparison with Chlorine Gas
While chlorine gas (Cl₂) is also used for disinfection, hypochlorous acid is considered safer and more effective in many scenarios. Chlorine gas requires careful handling due to its toxic nature, whereas hypochlorous acid can be generated on-site and used in a diluted form, minimizing risks.
Applications of Hypochlorous Acid
The versatility of hypochlorous acid is evident in its wide range of applications. From healthcare to agriculture, this compound has proven to be indispensable in maintaining hygiene and safety. Below are some of the most common uses of hypochlorous acid:
- Healthcare: Used for disinfecting medical equipment, surfaces, and wounds.
- Water Treatment: Effective in purifying drinking water and treating wastewater.
- Food Industry: Employed for sanitizing food processing equipment and surfaces.
- Agriculture: Utilized for disinfecting irrigation systems and controlling plant diseases.
Emerging Applications
Recent advancements have expanded the use of hypochlorous acid in areas such as air purification and personal care products. Its non-toxic and eco-friendly nature makes it a preferred choice for applications where safety and environmental impact are critical considerations.
How Hypochlorous Acid is Produced
Hypochlorous acid can be produced through several methods, the most common being the electrolysis of a saltwater solution. This process involves passing an electric current through a solution of sodium chloride (table salt) and water, resulting in the generation of hypochlorous acid and sodium hydroxide. This method is widely used due to its simplicity and cost-effectiveness.
Another method involves the reaction of chlorine gas with water, producing a mixture of hypochlorous acid and hydrochloric acid. While effective, this method requires careful handling of chlorine gas, making it less favorable compared to electrolysis.
On-Site Generation
On-site generation of hypochlorous acid has gained popularity due to its convenience and safety. By producing the acid as needed, users can avoid the risks associated with storing and transporting hazardous chemicals. This approach is particularly beneficial in remote or resource-limited settings.
Safety Considerations and Handling
While hypochlorous acid is generally considered safe, proper handling and storage are essential to ensure its effectiveness and minimize risks. Below are some key safety considerations:
- Storage: Store in a cool, dark place to prevent decomposition.
- Handling: Use protective gloves and eyewear when handling concentrated solutions.
- Disposal: Dispose of unused solutions according to local regulations to avoid environmental contamination.
Health Risks
Although hypochlorous acid is non-toxic at low concentrations, prolonged exposure to high concentrations can cause skin and respiratory irritation. It is important to follow safety guidelines and use appropriate personal protective equipment when working with this compound.
Environmental Impact of Hypochlorous Acid
Hypochlorous acid is considered an environmentally friendly disinfectant due to its biodegradability and minimal ecological impact. Unlike other chemical disinfectants, it breaks down into harmless byproducts such as water and salt, making it safe for use in sensitive ecosystems.
However, improper disposal or excessive use can lead to the formation of chlorinated organic compounds, which may have adverse effects on aquatic life. To mitigate these risks, it is important to adhere to recommended usage guidelines and ensure proper waste management practices.
Comparison with Other Disinfectants
When compared to other disinfectants such as bleach (sodium hypochlorite) and hydrogen peroxide, hypochlorous acid stands out for its effectiveness, safety, and eco-friendliness. Below is a comparison of these disinfectants based on key criteria:
- Effectiveness: Hypochlorous acid is more effective at lower concentrations compared to bleach.
- Safety: Unlike bleach, hypochlorous acid is non-corrosive and safe for use on sensitive surfaces.
- Environmental Impact: Hypochlorous acid breaks down into harmless byproducts, whereas bleach can release harmful chlorinated compounds.
Cost Considerations
While hypochlorous acid may have a higher upfront cost compared to traditional disinfectants, its efficiency and reduced need for protective equipment often result in long-term cost savings. Additionally, its on-site generation capabilities can further reduce costs associated with transportation and storage.
Recent Research and Developments
Ongoing research continues to uncover new applications and improvements in the production and use of hypochlorous acid. Recent studies have explored its potential in combating antibiotic-resistant bacteria, enhancing its role in healthcare and public health initiatives. Innovations in electrolysis technology have also led to more efficient and scalable production methods, making hypochlorous acid more accessible than ever before.
Future Prospects
The future of hypochlorous acid looks promising, with potential breakthroughs in areas such as antimicrobial coatings, advanced wound care, and sustainable agriculture. As awareness of its benefits grows, so too will its adoption across various industries.
Conclusion and Call to Action
In conclusion, the hypochlorous acid chemical formula represents a compound of immense importance and versatility. From its role in disinfection to its applications in healthcare and environmental protection, hypochlorous acid continues to demonstrate its value in modern society. Its unique properties, safety profile, and eco-friendly nature make it a preferred choice for a wide range of applications.
We encourage you to share your thoughts and experiences with hypochlorous acid in the comments section below. If you found this article informative, please consider sharing it with others who may benefit from this knowledge. For more insights into chemistry and related topics, explore our other articles on this site.