Table of Contents
- Introduction to Hypochlorous Acid
- Chemical Structure and Formula of Hypochlorous Acid
- How Hypochlorous Acid is Produced
- Applications of Hypochlorous Acid
- Hypochlorous Acid in Disinfection
- Health Benefits and Safety of Hypochlorous Acid
- Environmental Impact of Hypochlorous Acid
- Comparison with Other Disinfectants
- Recent Research and Developments
- Conclusion and Call to Action
Introduction to Hypochlorous Acid
Hypochlorous acid formula is a topic of immense importance in the field of chemistry, healthcare, and environmental science. This weak acid, represented by the chemical formula HOCl, is a powerful oxidizing agent with a wide range of applications. From disinfecting surfaces to treating wounds, hypochlorous acid plays a critical role in ensuring safety and hygiene.
Despite its simplicity, hypochlorous acid is a compound that has intrigued scientists for decades. Its ability to effectively kill bacteria, viruses, and fungi while being gentle on human tissues makes it a unique substance. Understanding the hypochlorous acid formula and its properties is essential for leveraging its potential in various industries.
In this article, we will delve deep into the chemistry of hypochlorous acid, its uses, and its benefits. Whether you're a chemist, a healthcare professional, or simply someone interested in learning more about this remarkable compound, this guide will provide you with all the information you need.
Read also:Kendall Jenner The Rise Of A Fashion Icon And Her Impact On The Industry
Chemical Structure and Formula of Hypochlorous Acid
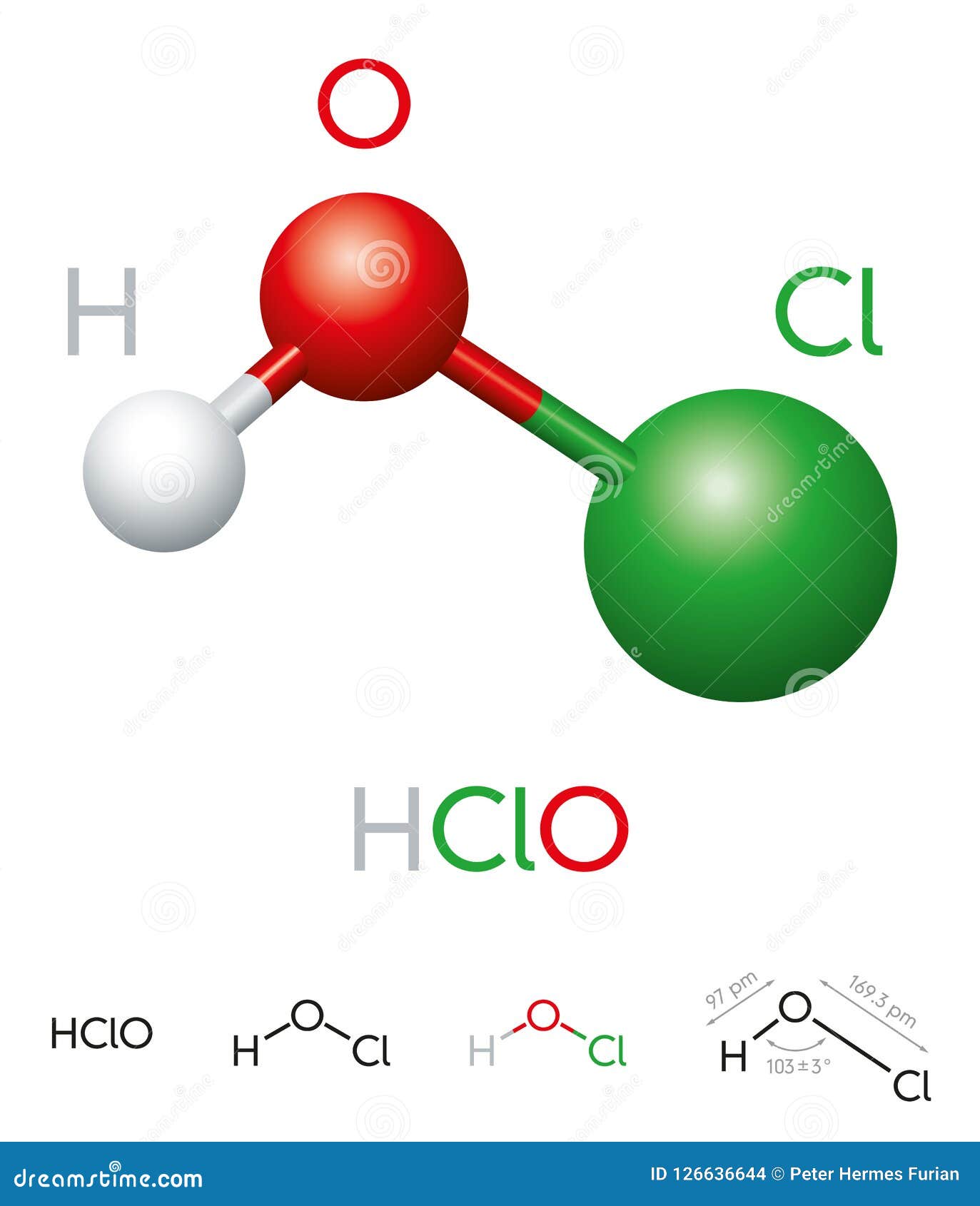
Hypochlorous acid is a simple molecule with a profound impact. Its chemical formula, HOCl, indicates that it is composed of one hydrogen atom, one oxygen atom, and one chlorine atom. This structure gives hypochlorous acid its unique properties and makes it highly reactive.
Understanding the Bonding in Hypochlorous Acid
The hypochlorous acid formula reveals that it is a covalent compound, where the atoms are bonded through shared electron pairs. The oxygen atom is bonded to both hydrogen and chlorine, forming a bent molecular geometry. This structure allows hypochlorous acid to act as a powerful oxidizing agent.
- Hydrogen: Provides the acidic property.
- Oxygen: Acts as the central atom, facilitating electron sharing.
- Chlorine: Contributes to the oxidizing power of the molecule.
Comparison with Other Chlorine Compounds
While hypochlorous acid is similar to other chlorine-based compounds like bleach (sodium hypochlorite), it is distinct in its molecular structure and behavior. Unlike bleach, which is highly alkaline, hypochlorous acid is a weak acid that exists in equilibrium with its dissociated ions in water.
How Hypochlorous Acid is Produced
Understanding how hypochlorous acid is produced is crucial for appreciating its widespread use. There are several methods for synthesizing hypochlorous acid, each suited to different applications.
Electrochemical Production
One of the most common methods for producing hypochlorous acid is through electrochemical processes. This involves passing an electric current through a solution of saltwater (sodium chloride) to generate chlorine gas, which then reacts with water to form hypochlorous acid.
The reaction can be summarized as follows:
Read also:Kid And His Mom Cctv Video Understanding The Impact And Importance Of Surveillance
- 2NaCl + 2H2O → Cl2 + 2NaOH
- Cl2 + H2O → HOCl + HCl
Chemical Synthesis
Another method involves the direct reaction of chlorine gas with water. This process is widely used in industrial settings to produce large quantities of hypochlorous acid for disinfection purposes.
Applications of Hypochlorous Acid
Hypochlorous acid has a wide range of applications across various industries. Its effectiveness as a disinfectant and its safety for human use make it a versatile compound.
Disinfection in Healthcare
In healthcare settings, hypochlorous acid is used to disinfect surfaces, medical equipment, and even wounds. Its ability to kill pathogens without causing harm to human tissues makes it an ideal choice for these applications.
Water Treatment
Hypochlorous acid is also used in water treatment plants to eliminate harmful microorganisms. Its effectiveness in neutralizing bacteria, viruses, and fungi ensures that drinking water is safe for consumption.
Hypochlorous Acid in Disinfection
The role of hypochlorous acid in disinfection cannot be overstated. It is one of the most effective and safest disinfectants available today.
Mechanism of Action
Hypochlorous acid works by oxidizing the cell walls of microorganisms, leading to their destruction. This process is highly efficient and occurs rapidly, making it an excellent choice for disinfection.
Advantages Over Traditional Disinfectants
Compared to traditional disinfectants like bleach, hypochlorous acid is less corrosive and does not leave harmful residues. This makes it suitable for use in environments where safety is a priority.
Health Benefits and Safety of Hypochlorous Acid
One of the key reasons for the popularity of hypochlorous acid is its safety for human use. Unlike many other disinfectants, it does not cause irritation or harm when used correctly.
Use in Wound Care
Hypochlorous acid is increasingly being used in wound care due to its ability to kill bacteria without damaging healthy tissues. This makes it an excellent choice for treating infections and promoting healing.
Skin and Eye Safety
Studies have shown that hypochlorous acid is safe for use on the skin and eyes, making it suitable for a wide range of applications, including personal hygiene products.
Environmental Impact of Hypochlorous Acid
Hypochlorous acid is not only effective but also environmentally friendly. Its breakdown products are harmless, and it does not contribute to pollution.
Biodegradability
Unlike many chemical disinfectants, hypochlorous acid breaks down into water and salt, leaving no harmful residues. This makes it a sustainable choice for disinfection.
Reduced Chemical Waste
By using hypochlorous acid, industries can significantly reduce their chemical waste, contributing to a cleaner and healthier environment.
Comparison with Other Disinfectants
When compared to other disinfectants, hypochlorous acid stands out for its effectiveness, safety, and environmental friendliness.
Effectiveness Against Pathogens
Hypochlorous acid is highly effective against a wide range of pathogens, including bacteria, viruses, and fungi. Its oxidizing properties make it superior to many traditional disinfectants.
Safety for Human Use
Unlike bleach and other harsh chemicals, hypochlorous acid is safe for use around humans and animals. This makes it ideal for use in homes, schools, and healthcare facilities.
Recent Research and Developments
Recent advancements in the study of hypochlorous acid have opened up new possibilities for its use. Researchers are exploring its potential in areas such as food safety, agriculture, and even cancer treatment.
Food Safety Applications
Hypochlorous acid is being investigated as a safe and effective way to sanitize food products. Its ability to kill pathogens without affecting the taste or quality of food makes it an attractive option for the food industry.
Potential in Cancer Treatment
Some studies suggest that hypochlorous acid may have applications in cancer treatment due to its ability to target and destroy cancer cells. While more research is needed, this is an exciting area of exploration.
Conclusion and Call to Action
In conclusion, the hypochlorous acid formula represents a compound of immense value and versatility. From its chemical structure to its wide-ranging applications, hypochlorous acid is a substance that continues to impact various fields positively. Its effectiveness as a disinfectant, coupled with its safety for human use, makes it an invaluable tool in ensuring hygiene and health.
We encourage you to share your thoughts on this article in the comments section below. If you found this guide helpful, please consider sharing it with others who might benefit from this information. Additionally, explore more articles on our website to deepen your understanding of chemistry and its applications in everyday life.